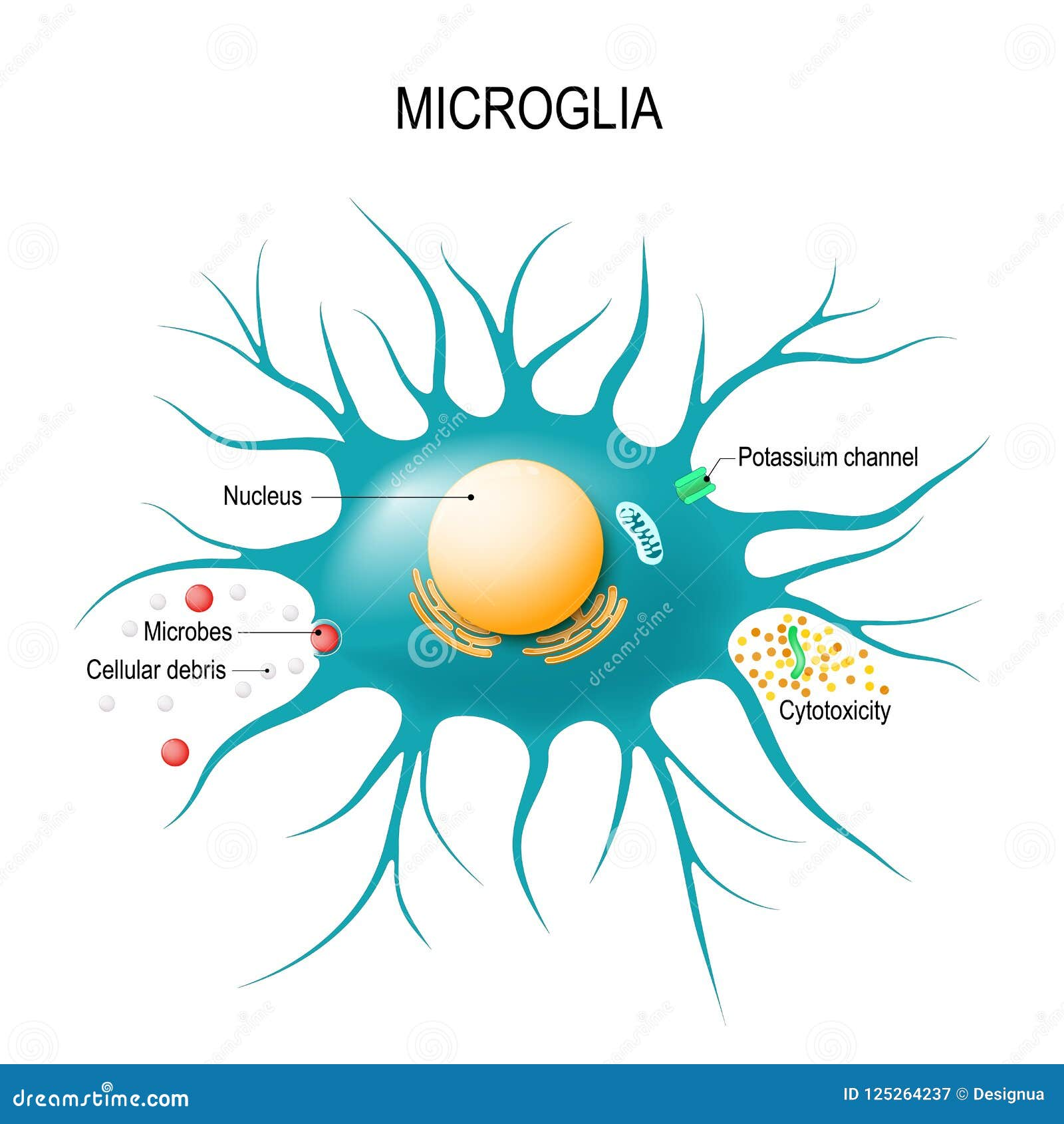
Microglial cells play a pivotal role in maintaining brain health, functioning as the brain’s immune system by constantly monitoring and responding to injury or disease. These remarkable cells are not only responsible for clearing out dead or damaged neurons, but they also engage in synaptic pruning, which is essential for the healthy transmission of information among neurons. Recent research by Beth Stevens, an eminent neuroscientist at Boston Children’s Hospital, sheds light on how abnormalities in microglial function can contribute to Alzheimer’s disease and other neurodegenerative diseases. This groundbreaking work emphasizes the importance of understanding microglial dynamics in the context of brain disorders, especially as the prevalence of Alzheimer’s disease continues to rise among the aging population. By fostering insights into these critical brain cells, Stevens and her team hope to pave the way for innovative treatments that can enhance the lives of millions affected by neurodegenerative conditions.
In the realm of neuroscience, glial cells, particularly microglia, are increasingly recognized for their essential function in the brain’s defense and repair mechanisms. These specialized cells are integral to the brain’s immune system, responding to threats and maintaining the delicate balance necessary for cognitive health. Research led by figures like Beth Stevens has illuminated how these brain guardians participate in synaptic remodeling, a process crucial for neuron communication. Moreover, the emerging understanding of how dysfunctional microglial activity can lead to conditions such as Alzheimer’s highlights the critical need for comprehensive studies in this area. As we delve deeper into the world of brain immunity and its implications for neurodegenerative disorders, the study of microglial cells promises to unveil transformative insights that could revolutionize treatment approaches.
The Role of Microglial Cells in Alzheimer’s Disease
Microglial cells are essential components of the brain’s immune system, constantly monitoring the neural environment for signs of inflammation or injury. Their role in maintaining brain health cannot be overstated, as these cells are responsible for clearing out debris, dead neurons, and other potentially harmful substances. In conditions like Alzheimer’s disease, the process can go awry, leading to excessive synaptic pruning that may contribute to memory loss and cognitive decline. The research led by Beth Stevens highlights the crucial balance microglial cells must maintain to protect the brain from neurodegenerative diseases.
Stevens’ groundbreaking work shows that when microglia misfire, such as during abnormal synaptic pruning, it can exacerbate the onset of Alzheimer’s disease, Huntington’s disease, and other related disorders. By examining how these immune cells interact with neurons, Stevens’ lab provides insights into how improper brain cell maintenance can lead to severe cognitive impairments. This new understanding offers potential pathways for developing biomarkers and therapeutic strategies aimed at mitigating the impacts of Alzheimer’s disease and enhancing the quality of life for millions.
Neurodegenerative Diseases and the Brain Immune System
Neurodegenerative diseases, including Alzheimer’s, pose a significant challenge to modern medicine, emphasizing the essential role of the brain’s immune system. Research has demonstrated that neuroinflammation plays a pivotal role in many of these conditions, highlighting the need for a deeper understanding of how the brain’s innate immune responses can influence disease progression. Through studies conducted in labs like that of Beth Stevens, significant correlations have been identified between microglial activation and the pathological features of Alzheimer’s disease, paving the way for new investigative approaches.
The balance between neuroprotection and neurotoxicity orchestrated by microglial cells serves as a critical point of research. Understanding the mechanisms behind this duality can reveal how neurodegenerative processes like synaptic pruning are regulated. Stevens’ work points toward a future where fine-tuning the brain’s immune responses could yield therapies that preemptively address the onset or progression of conditions like Alzheimer’s. By targeting the underlying inflammatory pathways, researchers hope to develop groundbreaking treatments that not only halt further damage but also restore cognitive function.
Synaptic Pruning: Defining Connections in the Brain
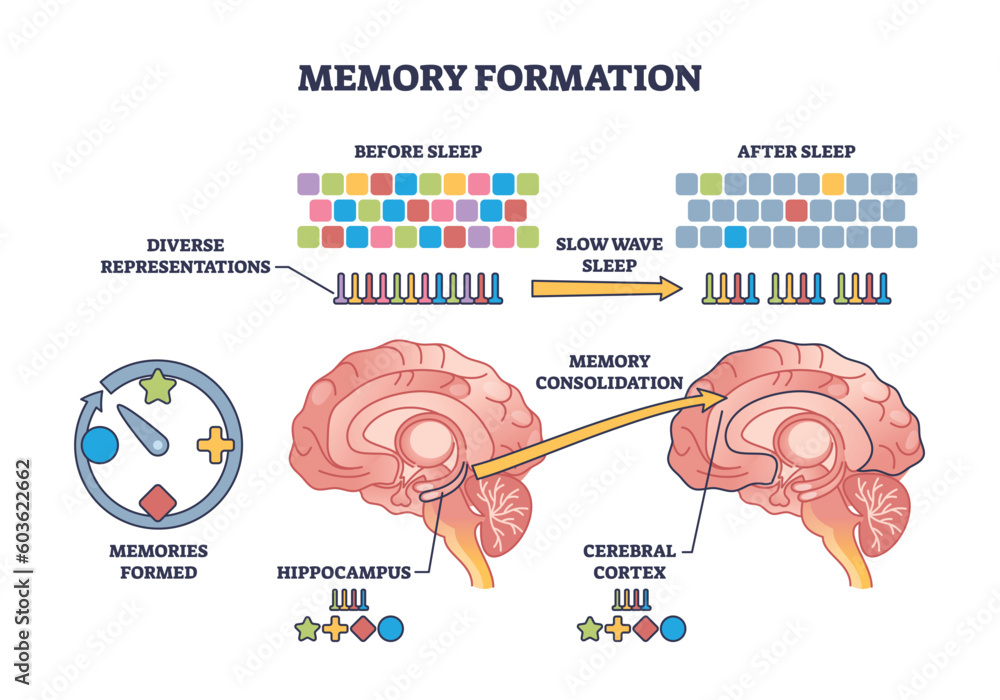
Synaptic pruning is a vital process in the development and functionality of neural circuits, ensuring that only the most efficient connections are retained in the brain. During development, microglial cells play an active role in pruning excess synapses, which is essential for cognitive growth and adaptability. However, the understanding that improper synaptic pruning can lead to neurodegenerative diseases like Alzheimer’s has sparked new interest in exploring this phenomenon. Beth Stevens’ work illustrates how disruptions in this process can result in cognitive dysfunction, and the importance of properly regulated pruning cannot be overstated.
Moreover, the implications of Stevens’ research extend beyond Alzheimer’s to a broader spectrum of neurodevelopmental disorders. By elucidating how microglial cells regulate synaptic pruning, researchers are recognizing potential interventions that could mitigate deleterious effects on neural connections. Future studies could lead to the development of strategies that harness the power of microglial cells to restore synaptic integrity and promote brain health, ultimately addressing the challenges posed by Alzheimer’s and other neurodegenerative diseases.
Beth Stevens’ Pioneering Research in Neurodegeneration
Beth Stevens has emerged as a leading figure in the realm of neurodegenerative disease research, particularly focusing on the function of microglial cells in synaptic health. Her dedication to understanding the brain’s immune responses has transformed traditional views on these cells, leading to innovative insights into conditions like Alzheimer’s disease. Stevens emphasizes how basic research can yield profound implications for treating complex disorders, advocating for the importance of curiosity-driven science in unlocking the mysteries of neurodegeneration.
Through her pioneering work, Stevens has contributed significantly to the identification of biomarkers for Alzheimer’s disease while also laying the groundwork for future therapeutic approaches. By exploring the ways microglia interact with neurons, her studies open avenues for targeted treatments that address the underlying mechanisms responsible for synaptic dysfunction. With each discovery, Stevens’ research not only enhances understanding of Alzheimer’s but also inspires a new generation of researchers committed to tackling the challenges of neurodegenerative diseases.
The Impact of Federal Funding on Alzheimer’s Research
Underpinning the progress in understanding Alzheimer’s disease and neurodegenerative disorders is the vital role of federal funding, as highlighted by Stevens in her journey as a researcher. Grants from the National Institutes of Health (NIH) have been instrumental in supporting investigations that probe the intricate workings of microglial cells and their implications in Alzheimer’s. This funding enables scientists to pursue innovative research questions that might not otherwise receive attention, fostering an environment where groundbreaking discoveries can flourish.
Stevens credits her accomplishments to the sustained support provided by federal agencies, which allow researchers to explore the complexities of the brain’s immune system without the immediate pressure of direct clinical applications. This long-term vision is necessary for unraveling the nuances of neurodegeneration, as understanding fundamental biological processes often lays the groundwork for developing effective treatments. The continued investment in Alzheimer’s research signifies a commitment to addressing the growing impact of this disease on public health.
Developing New Biomarkers for Alzheimer’s Disease
As research evolves, the quest for reliable biomarkers for Alzheimer’s disease intensifies. Biomarkers serve as critical tools for early diagnosis and management of neurodegenerative diseases, providing insight into the disease’s progression. Stevens’ findings on the role of microglial cells in synaptic pruning have implications for identifying biomarkers that could signal the onset of Alzheimer’s before significant cognitive decline occurs. Establishing such indicators holds the potential to revolutionize patient care and alter treatment protocols.
By focusing on the relationship between microglia and synaptic health, Stevens is paving the way for novel diagnostic approaches that could detect maladaptive pruning patterns long before clinical symptoms become apparent. This proactive strategy could facilitate earlier interventions, ultimately improving patient outcomes and potentially halting the progression of Alzheimer’s disease. The development of such biomarkers is a testament to the interconnectedness of basic science and clinical application in the fight against neurodegenerative diseases.
The Intersection of Basic Science and Clinical Application
The journey from laboratory research to clinical application is often prolonged, yet understanding this relationship is crucial for addressing complex disorders like Alzheimer’s disease. Stevens emphasizes that foundational research in neuroimmunology has significant implications in clinical settings, showcasing how insights gained from microglial studies can lead to advancements in treatment strategies. Progress in basic science cultivates the understanding necessary for developing therapeutic approaches targeting the root causes of neurodegenerative diseases.
Bridging the gap between these two realms requires not only innovative scientific inquiry but also collaboration across disciplines. By fostering cross-disciplinary partnerships, researchers can ensure that discoveries related to microglial function and synaptic health translate into effective treatment regimes for Alzheimer’s. This comprehensive approach is essential for developing therapies that can alter the course of neurodegeneration and enhance the lives of those affected by these debilitating diseases.
Addressing the Challenges of Neuroinflammation
Neuroinflammation has emerged as a pivotal factor in the progression of many neurodegenerative diseases, including Alzheimer’s. The activation of microglial cells often leads to an inflammatory response that can further exacerbate neuronal damage and synaptic dysfunction. Understanding how to effectively modulate this inflammatory response represents a significant challenge for researchers like Beth Stevens, who are working to untangle the complex roles of these immune cells in brain health and disease.
Addressing neuroinflammation involves not only identifying the triggers that activate microglia but also exploring potential therapeutic interventions that can calm this overactive immune response. Stevens’ research offers hope for future strategies that might subdue neuroinflammation without diminishing the protective functions that microglial cells provide. This delicate balance is key to developing novel therapies that can reduce the impact of neurodegenerative diseases while preserving cognitive function and quality of life.
Future Directions in Alzheimer’s Research
The future of Alzheimer’s research looks promising, with advancements in understanding microglial cell function leading the charge. Stevens’ work demonstrates that by harnessing the brain’s immune responses, researchers can create innovative strategies to combat neurodegenerative diseases. Key to this progress is the commitment to ongoing research and collaboration within the scientific community, which is essential for tackling the complexities associated with Alzheimer’s.
Moving forward, researchers are poised to explore several promising avenues: refining therapeutic approaches that engage microglial cells, developing early diagnostic tools based on neuroinflammatory markers, and identifying lifestyle or pharmaceutical interventions that could enhance brain health. Each step taken in this direction represents a potential breakthrough in understanding and treating Alzheimer’s disease, as the integration of basic science and clinical insights becomes increasingly pivotal in reshaping patient care.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells act as the brain’s immune system, monitoring for signs of illness or injury. In Alzheimer’s disease, they are crucial for clearing out dead cells and toxic plaques. However, abnormal activation of microglial cells can lead to excessive synaptic pruning, which may contribute to neurodegenerative processes observed in Alzheimer’s.
How do microglial cells contribute to synaptic pruning in the brain?
Microglial cells facilitate synaptic pruning by removing unnecessary synapses during brain development and in response to injury. This process is essential for normal brain function, but dysregulation of microglial activity can result in excessive synaptic loss, which is a potential factor in neurodegenerative diseases such as Alzheimer’s.
What is the significance of Beth Stevens’ research on microglial cells?
Beth Stevens’ research has transformed our understanding of microglial cells and their role in neurodegenerative diseases. Her work highlights the dual role of these cells in maintaining brain health, as they help both to sculpt neural circuits through synaptic pruning and to clear away damaged neurons. This insight is particularly important for developing new treatments for diseases like Alzheimer’s.
Can microglial cells influence the progression of neurodegenerative diseases?
Yes, microglial cells significantly influence the progression of neurodegenerative diseases such as Alzheimer’s. While they normally protect the brain by removing debris and promoting healing, dysfunctional microglial activity can lead to increased inflammation and aberrant synaptic pruning, exacerbating disease symptoms and progression.
What is the link between microglial cells and the brain’s immune response?
Microglial cells are a central component of the brain’s immune system. They respond to injury and infection by activating inflammatory pathways, which can help in healing but may also lead to damage if misregulated. In the context of Alzheimer’s disease, their role in immune response and synaptic pruning is critical for understanding disease mechanisms.
How does microglial dysfunction relate to Huntington’s disease?
Research shows that microglial dysfunction is not only relevant to Alzheimer’s disease but also to Huntington’s disease. Aberrant microglial activation can contribute to neuroinflammation and synaptic dysfunction, similar to what is seen in Alzheimer’s, thus playing a key role in the pathology of multiple neurodegenerative conditions.
What are potential therapeutic targets based on microglial cell research?
Therapeutic targets identified from microglial cell research include pathways that regulate their activation and function. By modulating microglial activity, it may be possible to restore normal synaptic pruning processes and mitigate the neuroinflammation associated with Alzheimer’s disease and other neurodegenerative disorders.
How can understanding microglial cells aid in developing biomarkers for neurodegenerative diseases?
Understanding the role of microglial cells in neurodegenerative diseases offers insights for developing new biomarkers. Abnormal changes in microglial activity and function might be detectable early in diseases like Alzheimer’s, leading to improved diagnosis and treatment monitoring.
What are the implications of microglial cell research for Alzheimer’s disease care?
Microglial cell research has significant implications for Alzheimer’s disease care. By unraveling the mechanisms by which these cells contribute to disease progression, scientists like Beth Stevens aim to develop targeted therapies and interventions that could improve outcomes for millions affected by Alzheimer’s.
| Key Points |
|---|
| Microglial cells are the brain’s immune system, responsible for patrolling for illness or injury. |
| They help clear dead or damaged cells and prune synapses to maintain neural health. |
| Aberrant pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Research led by Beth Stevens has opened pathways for new biomarkers and treatments for Alzheimer’s disease. |
| This research is supported by federal agencies, notably the National Institutes of Health (NIH), which have been crucial in its development. |
| Vital discoveries regarding microglial function emerged from curiosity-driven basic science. |
Summary
Microglial cells play a crucial role in maintaining brain health by functioning as the brain’s immune system. Their ability to clear damaged cells and prune synapses is vital for normal neuronal function. However, when this process becomes dysregulated, it can lead to devastating neurodegenerative diseases such as Alzheimer’s and Huntington’s. Under the guidance of researchers like Beth Stevens, the ongoing exploration of microglial functions is paving the way for new diagnostics and treatments, showcasing the importance of persistent research in understanding and addressing these complex brain disorders.