TIM-3 therapy for Alzheimer’s is forging a new path in the realm of Alzheimer’s disease treatment by leveraging insights from immune system therapy traditionally used in cancer treatments. This innovative approach aims to tackle the underlying challenges faced by microglia, the brain’s immune cells, which become dysfunctional in the presence of amyloid plaques. A groundbreaking study highlighted that by deleting or blocking the TIM-3 gene, these immune cells are freed to effectively clear harmful plaques, potentially restoring cognitive function and improving memory in models of Alzheimer’s. The implications of this research could be transformative, particularly as it explores how cancer therapy repurposed can offer new hope for patients suffering from this debilitating condition. As researchers continue to unravel the connections between microglia and Alzheimer’s, TIM-3 therapy stands out as a promising candidate that could redefine treatment paradigms for millions affected by this neurodegenerative disease.
Exploring the potential of TIM-3 for Alzheimer’s disease reveals an intersection of immunotherapy and neurology that could significantly reshape our understanding of neurodegenerative disorders. Alternative strategies, such as using checkpoint inhibition, leverage immune system mechanisms to combat the damaging effects of plaque accumulation associated with cognitive decline. The role of microglia, often described as the custodians of brain health, is pivotal; when their functionality is compromised by the overexpression of certain genes like HAVCR2, memory and cognitive clarity are at stake. This correlation between immune responses and cognitive health not only highlights exciting avenues for research but also emphasizes the importance of exploring treatments that engage the immune system more effectively. By harnessing insights from cancer therapeutics, we may unlock innovative ways to alleviate the burdens of Alzheimer’s disease, providing hope for enhanced quality of life for affected individuals.
Overview of TIM-3 and Alzheimer’s Disease
Alzheimer’s disease (AD) primarily affects aging populations, particularly through its late-onset form, which accounts for 90-95% of cases. Recent research has revealed that TIM-3, an immune checkpoint molecule, is linked to late-onset Alzheimer’s, acting as a genetic risk factor. The implications of this connection are immense, as understanding TIM-3’s role could pave the way for novel treatment strategies that leverage immune system mechanisms to combat AD.
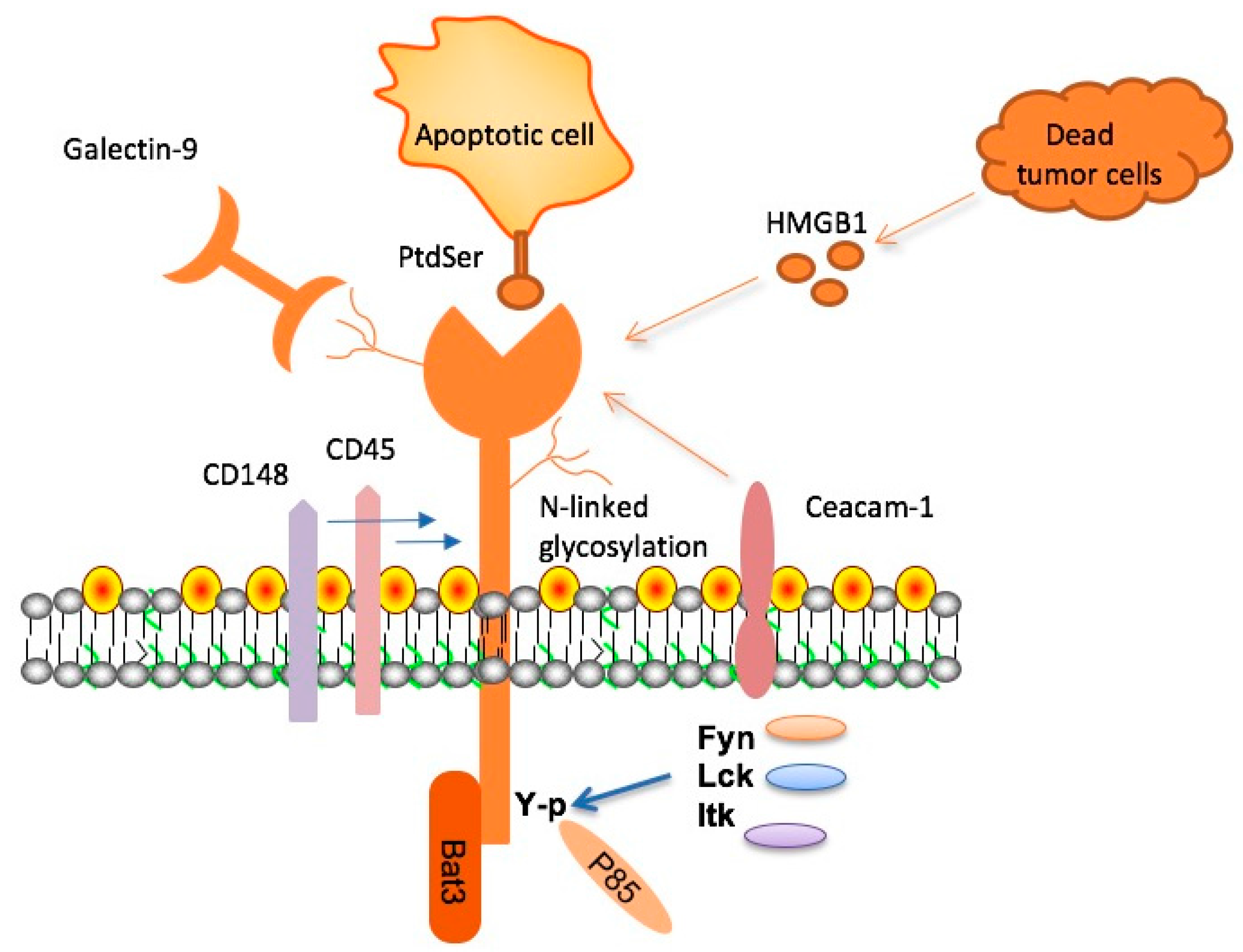
TIM-3, or T-cell immunoglobulin mucin 3, serves a dual purpose in the immune response, regulating T cell activity to prevent overreaction to infections. However, in the context of Alzheimer’s, TIM-3’s inhibitory function limits microglial activity—the brain’s immune cells—in clearing amyloid plaques, which are characteristic of the disease. This discovery suggests that therapeutic strategies targeting TIM-3 could potentially restore the brain’s ability to eliminate harmful plaque accumulation.
The Role of Microglia in Alzheimer’s Disease
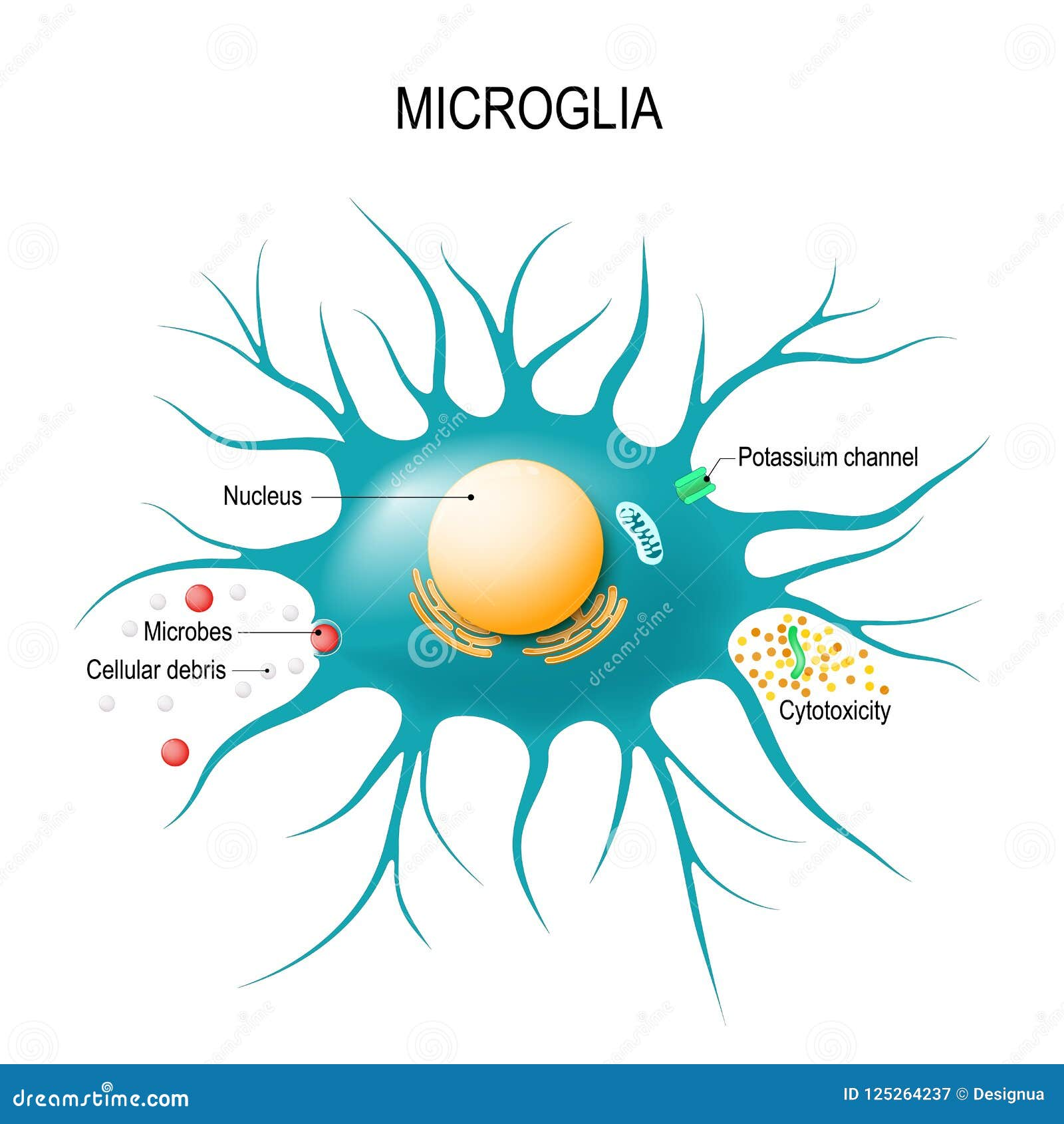
Microglia are essential immune cells in the brain that maintain homeostasis and support neuronal functions. In healthy conditions, these cells prune unnecessary synapses to facilitate memory and learning. However, in Alzheimer’s disease, the upregulation of TIM-3 inhibits microglial functions, particularly their ability to phagocytose amyloid plaques. This impediment results in plaque accumulation, contributing to cognitive decline, hence underscoring the importance of microglial activity in Alzheimer’s pathology.
Furthermore, the developmental aspect of microglial function demonstrates the complexity of their role. While their pruning capabilities are crucial during brain development, a robust expression of TIM-3 in older adults with Alzheimer’s disease prevents microglia from performing necessary cleanup tasks. Understanding how to manipulate TIM-3 levels may be key in harnessing microglial functions to clear plaque and maintain cognitive health.
TIM-3 Therapy for Alzheimer’s: A Promising Avenue
Research into TIM-3 therapy for Alzheimer’s disease suggests that blocking this checkpoint molecule could unleash microglial activity, allowing these immune cells to clear amyloid plaques more effectively. By genetically deleting TIM-3 in lab mice, researchers observed improved cognitive functions, indicating that similar strategies could yield beneficial outcomes in humans. The prospect of repurposing existing anti-TIM-3 antibodies, initially developed for cancer therapy, could fast-track the development of new Alzheimer’s treatments.
Future therapies could involve administering anti-TIM-3 antibodies or small molecules designed to inhibit TIM-3’s actions. This targeted approach may not only enhance microglial clearance of plaques but also mitigate cognitive impairments commonly associated with Alzheimer’s disease. As research progresses, the translation of TIM-3 therapies into clinical settings holds great potential for altering the disease’s trajectory and improving patient outcomes.
Exploring Immunotherapy in Alzheimer’s Treatment
Immunotherapy, initially hailed for its success in cancer treatment, is being evaluated for its potential application in Alzheimer’s disease. The underlying principle involves harnessing the body’s immune system to combat disease processes, such as the amyloid plaque formation seen in AD. By understanding checkpoint molecules like TIM-3, researchers can develop immunomodulatory strategies that may enhance the ability of the immune system to tackle Alzheimer’s pathology more effectively.
The concept of repurposing cancer therapies for Alzheimer’s unlocks a significant opportunity for innovation in AD treatment. Given that TIM-3 is already a well-studied checkpoint target in cancer immunotherapy, leveraging this knowledge could streamline the process of developing new treatments for Alzheimer’s. This cross-disciplinary approach may not only accelerate research but also uncover novel mechanisms to promote brain health against degenerative diseases.
Mechanisms of TIM-3 Action in the Brain
The TIM-3 gene influences immune regulation within the brain, particularly affecting microglial functionality and their interaction with amyloid plaques. High expression levels of TIM-3 on microglia can lead to decreased phagocytic activity, resulting in plaque persistence and the associated cognitive decline seen in Alzheimer’s disease. By elucidating the specific mechanisms through which TIM-3 operates, scientists can begin to target this pathway therapeutically to enhance plaque clearance.
Understanding the mechanisms behind TIM-3’s inhibition of microglial activity opens new avenues for research into Alzheimer’s treatment. For instance, inhibiting TIM-3 could reactivate microglia to perform their critical roles in plaque surveillance and clearance. This nuanced understanding of TIM-3’s dual role in immune regulation and Alzheimer’s may lead to novel therapeutic strategies that facilitate microglial accessibility to pathogenic amyloid deposits.
Future Directions in Alzheimer’s Research
As researchers continue to explore the role of TIM-3 in Alzheimer’s disease, several future directions appear promising. One key aspect involves investigating how different polymorphisms of the TIM-3 gene influence individual susceptibility to Alzheimer’s and the effectiveness of potential therapies. By identifying these genetic variations, personalized medicine approaches could be developed, tailoring treatments based on an individual’s genetic makeup.
Moreover, ongoing studies utilizing models that express the human TIM-3 gene may provide deeper insights into the translatability of findings from animal studies to human subjects. Ultimately, understanding how TIM-3 influences not only immune cell behavior but also the broader neurological environment will be crucial in developing comprehensive strategies to combat Alzheimer’s disease.
Challenges in Alzheimer’s Drug Development
Despite promising leads, the pathway to effective Alzheimer’s treatment remains riddled with challenges. Many drug candidates targeting amyloid beta have failed in clinical trials, often revealing only marginal cognitive improvements. The complexity of Alzheimer’s disease necessitates a reevaluation of current therapeutic approaches and the mechanisms by which they operate, particularly in relation to TIM-3.
Moreover, factors such as the blood-brain barrier and varying expressions of targets like TIM-3 across individuals can complicate drug efficacy. Overcoming these hurdles is critical in the development of TIM-3-based therapies and ensuring they achieve optimal effects in clearing plaques and enhancing cognitive functions.
The Importance of Collaborative Research
Collaborative efforts between institutions are vital for advancing Alzheimer’s research, particularly in exploring innovative therapies like TIM-3 modulation. The shared expertise of laboratories, such as those led by prominent researchers at Harvard, allows for diverse approaches to studying complex biological questions regarding microglial functions.
By pooling resources and knowledge, collaborative teams can accelerate the discovery of viable therapeutic pathways and enhance the understanding of the underlying biology of Alzheimer’s disease. Such partnerships could ultimately expedite the translation of laboratory discoveries into meaningful clinical interventions, providing hope to millions affected by Alzheimer’s.
Timelines and Expectations for TIM-3 Therapy
The journey from basic research to clinical application often spans several years, and the investigation into TIM-3 therapy for Alzheimer’s is no exception. Current trials are focused on confirming the effectiveness of TIM-3 modulation in vivo and determining optimal dosing regimens that will ensure safety and efficacy in humans. Rigorous preclinical studies are paramount to gather sufficient data for advancement to human trials.
As researchers aim to establish a timeline for potential treatment options, the focus will be on not only the mechanistic understanding of TIM-3 but also the developmental trajectories of its therapeutic application. Continued investigation and adaptive trial designs will be key in bringing innovative solutions to the forefront of Alzheimer’s disease management.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s disease and how does it work?
TIM-3 therapy for Alzheimer’s disease involves targeting the TIM-3 molecule, which inhibits microglia (the immune cells in the brain) from clearing amyloid plaques. By blocking TIM-3, researchers have found that microglia can be activated to attack and remove these plaques, potentially restoring cognitive functions and memory.
How effective is TIM-3 therapy in reversing Alzheimer’s disease symptoms?
Research using TIM-3 therapy has shown promising results in animal models, with mice exhibiting improved memory and behavior after TIM-3 was genetically deleted. These findings suggest that TIM-3 therapy could enhance plaque clearance and cognitive function, although human trials are still needed to evaluate its effectiveness.
Is TIM-3 therapy for Alzheimer’s a repurposed cancer therapy?
Yes, TIM-3 therapy for Alzheimer’s is derived from an immune system strategy that was initially developed for cancer treatment. The same checkpoint molecules, including TIM-3, that are used to inhibit anti-tumor responses are now being explored for their potential to rejuvenate the immune response against amyloid plaques in Alzheimer’s disease.
What role do microglia play in Alzheimer’s and how does TIM-3 impact their function?
Microglia are essential for maintaining brain health, including clearing amyloid plaques associated with Alzheimer’s disease. TIM-3 inhibits microglia’s ability to perform this function, leading to plaque accumulation. TIM-3 therapy aims to reverse this inhibition, allowing microglia to effectively clear plaques and potentially restore cognitive function.
What are the current research developments regarding TIM-3 therapy for Alzheimer’s disease?
Recent studies highlight that blocking TIM-3 in animal models leads to enhanced plaque clearance and cognitive improvement. Ongoing research is focused on testing humanized anti-TIM-3 antibodies in mouse models to further understand the therapy’s potential and develop effective treatments for Alzheimer’s disease.
What genetic factors are associated with TIM-3 and Alzheimer’s disease?
The TIM-3 gene, known as HAVCR2, has been linked to a higher genetic risk for late-onset Alzheimer’s disease. Certain polymorphisms of the TIM-3 gene result in its increased expression on microglia, contributing to the inhibition of plaque clearance which exacerbates Alzheimer’s progression.
What are the implications of TIM-3 therapy for future Alzheimer’s treatments?
TIM-3 therapy represents a novel approach in Alzheimer’s disease treatment, potentially offering insights into immune system interactions in the brain. If successful, it could pave the way for more effective therapies by leveraging existing anti-TIM-3 antibodies, moving beyond traditional amyloid-targeting strategies.
Are there any risks associated with TIM-3 therapy for Alzheimer’s disease?
As with any therapy targeting immune responses, there could be risks involved, such as the potential for autoimmune reactions or unintended effects on normal brain function. Comprehensive clinical trials are necessary to thoroughly assess the safety and efficacy of TIM-3 therapy for Alzheimer’s disease.
| Key Point | Details |
|---|---|
| TIM-3 Role | TIM-3 is an immune checkpoint molecule that inhibits microglia from clearing Alzheimer’s plaques. |
| Research Findings | Deleting TIM-3 expression in mice improved plaque clearance and restored cognitive functions. |
| Late-Onset Alzheimer’s | 90-95% of Alzheimer’s cases are late-onset; TIM-3 has been linked to genetic risk factors for the disease. |
| Microglia Function | Microglia are brain immune cells responsible for clearing plaques and memory function. Increased TIM-3 prevents them from doing so. |
| Potential Treatment | Therapy may involve anti-TIM-3 antibodies to inhibit the TIM-3 function, allowing microglia to clear plaques. |
| Future Directions | Researchers are testing human anti-TIM-3 in Alzheimer’s models to evaluate treatment efficacy. |
Summary
TIM-3 therapy for Alzheimer’s presents a promising new approach to combating the disease. By targeting the TIM-3 checkpoint molecule, researchers aim to enhance the ability of microglia to clear harmful plaques in the brain, potentially restoring cognitive function. This groundbreaking strategy is rooted in the successful use of immune checkpoint inhibitors in cancer therapy and marks a significant step forward in Alzheimer’s research, which has faced numerous challenges in previous drug trials.