Alzheimer’s research has taken a transformative leap forward, particularly through the groundbreaking work of neuroscientist Beth Stevens at Boston Children’s Hospital. Her exploration of microglial cells, the brain’s immune defenders, has unveiled crucial insights into how these cells affect neuronal health and connectivity. Stevens’ findings suggest that faulty pruning by microglia may play a significant role not only in Alzheimer’s disease but also in other neurodegenerative diseases such as Huntington’s disease. As the number of Americans affected by Alzheimer’s continues to rise, innovative research like Stevens’ is essential for developing new treatments and diagnostic biomarkers. By focusing on the interplay between neuroscience and immune processes in the brain, we stand at the forefront of a potential revolution in the fight against these debilitating disorders.
Exploring advancements in the study of cognitive decline, particularly age-related impairments, is vital for understanding the complex mechanisms behind Alzheimer’s disease. This inquiry into the brain’s immune system, specifically how microglial cells manage neuronal health, highlights the intricate relationship between brain function and neurodegenerative disorders. Researchers like Beth Stevens are illuminating pathways that may lead to effective interventions for conditions such as Huntington’s disease, showcasing the significance of foundational science in addressing challenging ailments. As we seek innovative solutions in this critical area of medical research, the importance of understanding these cellular processes cannot be overstated. The ongoing efforts to link immune response and brain health represent a promising frontier in our battle against memory loss and associated diseases.
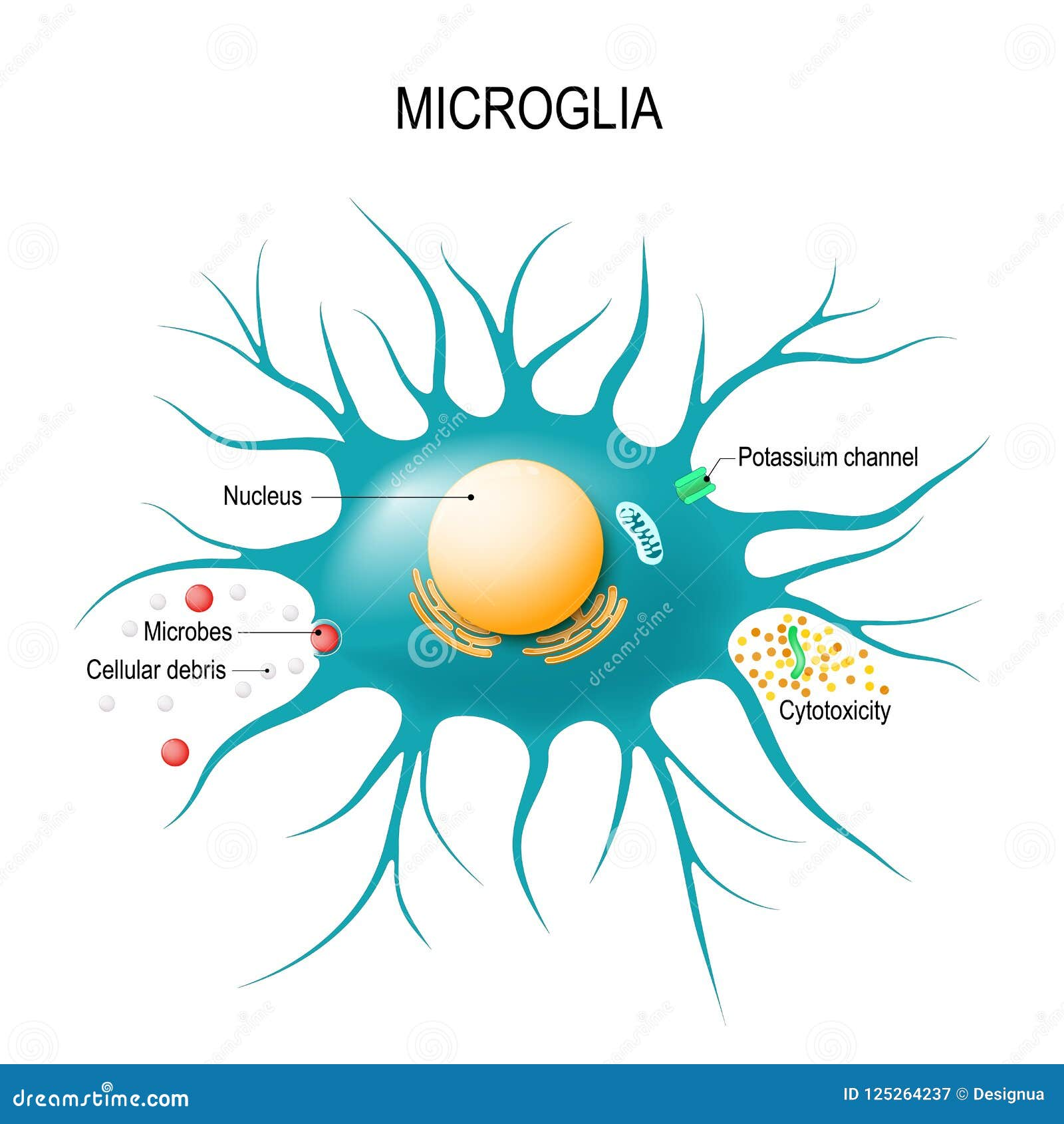
Understanding Microglial Cells in Neurodegenerative Diseases
Microglial cells serve a critical role in the brain’s immune system, and their functions are vital for maintaining neurological health. These specialized cells constantly monitor the brain’s environment, detecting signs of injury or disease. When activated, microglia help clear damaged neurons and participate in the pruning of synapses — the connections that enable communication between neurons. However, as research by Beth Stevens and her team demonstrates, improper functioning of microglia can lead to detrimental effects, potentially accelerating neurodegenerative diseases such as Alzheimer’s and Huntington’s disease.
Aberrant synaptic pruning carried out by microglial cells can remove healthy connections among neurons, leading to cognitive declines seen in conditions like Alzheimer’s disease. These findings revolutionize our understanding of neurodegenerative processes and highlight the intricate role of microglial cells in maintaining neuronal health. By further investigating the interactions between microglia and neurons, researchers can devise innovative therapeutic strategies aiming to restore balance and functionality in the brain’s immune responses.
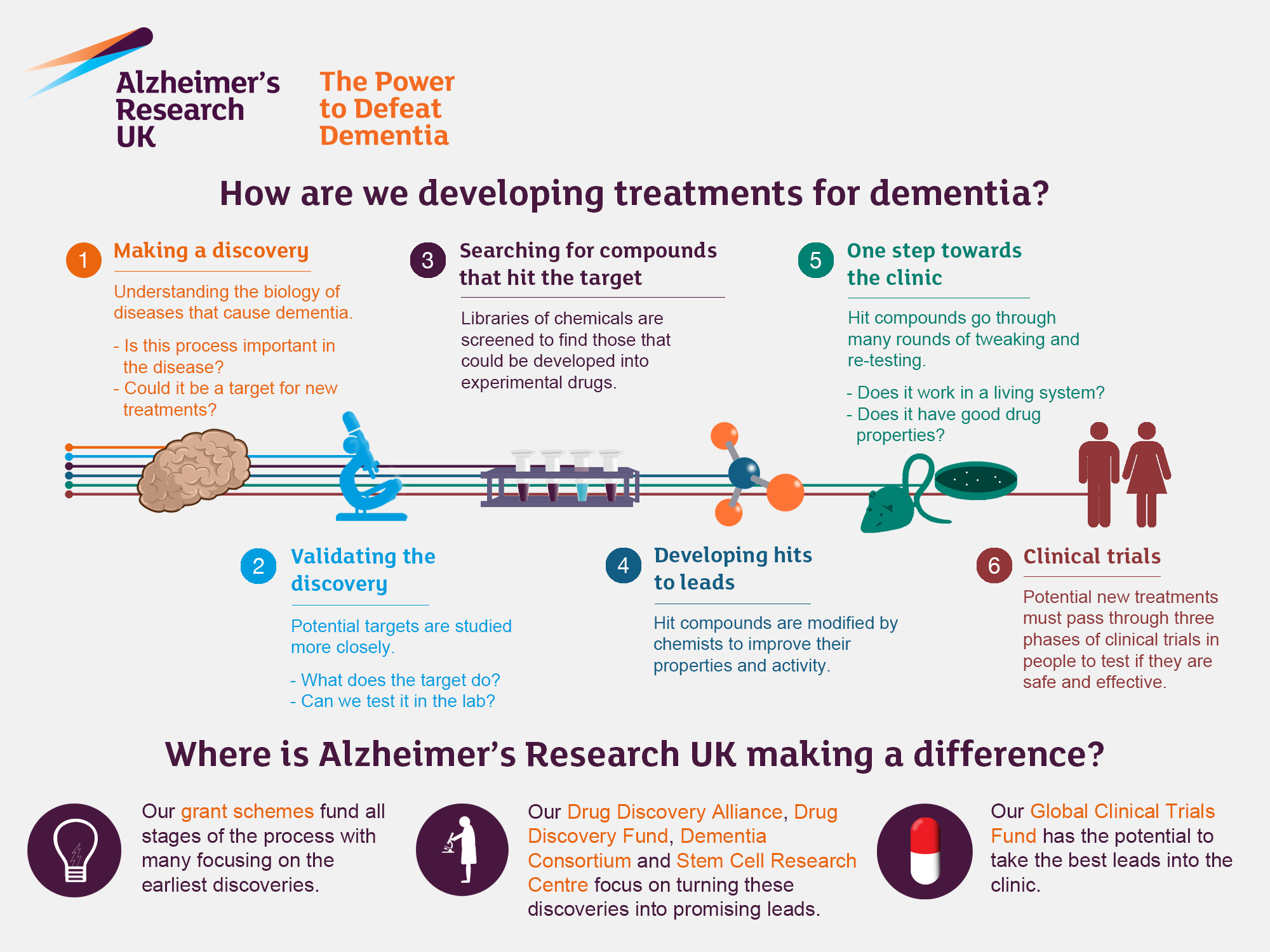
The Impact of Alzheimer’s Research on Treatment Approaches
Alzheimer’s research is at the forefront of developing new treatment strategies for neurodegenerative diseases. The groundbreaking work of scientists like Beth Stevens exemplifies how cellular mechanisms can unveil new pathways for intervention. By identifying the role of microglial cells in Alzheimer’s pathology, researchers are setting the stage for novel medications that can target these immune cells and correct their malfunctioning processes. This approach not only holds promise for therapeutic advancements but also for the earlier detection of Alzheimer’s through the discovery of biomarkers.
As the population ages, the urgency for effective treatments grows. Alzheimer’s disease affects millions of Americans, and its prevalence is expected to soar in the coming decades. The research spearheaded by Stevens and her colleagues not only enhances the fundamental understanding of Alzheimer’s but also provides hope for a future where neurodegenerative diseases can be managed effectively. Innovative solutions derived from such research may ultimately improve the quality of life for countless individuals suffering from cognitive decline.
Exploring the Role of Microglia in Disease Progression
The study of microglial cells extends beyond Alzheimer’s, intersecting with various neurodegenerative diseases, including Huntington’s disease. These immune cells have the capability to influence neuroinflammation and neuronal survival, which are critical factors in disease progression. Beth Stevens’ research introduces pivotal insights into how microglia respond to pathological states and contribute to the deterioration of neural circuits. Understanding these dynamics is essential for developing therapies aimed specifically at modulating microglial activity.
As we delve deeper into the mechanisms by which microglial cells operate, it becomes increasingly clear that these cells can be both protectors and aggressors in the brain. The dual role of microglia in neurodegenerative diseases means that any therapeutic interventions must take a balanced approach, potentially harnessing their healing properties while mitigating their harmful effects. Ongoing research continues to reveal the complex relationship between microglial cells and neurodegeneration, paving the way for future advances in neuroscience.
Frequently Asked Questions
How do microglial cells contribute to Alzheimer’s research?
Microglial cells play a crucial role in Alzheimer’s research by acting as the brain’s immune system. They help clear out dead or damaged cells and prune synapses, which are essential for neuron communication. However, aberrant pruning by microglia has been shown to contribute to Alzheimer’s disease and other neurodegenerative diseases, highlighting the importance of understanding their functions in order to develop new treatments.
What is the significance of Beth Stevens’ work in understanding neurodegenerative diseases like Alzheimer’s?
Beth Stevens’ research has transformed our understanding of microglial cells and their role in neurodegenerative diseases such as Alzheimer’s and Huntington’s disease. By uncovering how improper synaptic pruning by these cells can lead to disease progression, Stevens’ work lays the groundwork for developing new biomarkers and therapies aimed at improving the lives of those affected by Alzheimer’s.
What advancements have been made in Alzheimer’s research through the study of microglial cells?
Recent advancements in Alzheimer’s research have identified that microglial cells can both protect and endanger brain health. Aberrant pruning by these cells is linked to the development of Alzheimer’s, highlighting the need for targeted therapies that address microglial function to prevent or slow disease progression.
How is federal funding impacting Alzheimer’s research conducted by labs like Beth Stevens’?
Federal funding is vital for advancing Alzheimer’s research, particularly in labs like Beth Stevens’. It provides essential resources that allow researchers to explore foundational questions about microglial cells and their role in neurodegenerative diseases. This funding supports curiosity-driven science, leading to transformative discoveries that can improve treatment approaches for Alzheimer’s and related conditions.
What future implications does Beth Stevens’ research on microglial cells have for Alzheimer’s treatment?
Beth Stevens’ research on microglial cells suggests a future where understanding their mechanisms can lead to the development of new treatments for Alzheimer’s disease. By identifying how these immune cells contribute to synaptic health, her findings can pave the way for innovative therapeutic strategies that could alter the course of the disease for millions affected by Alzheimer’s.
How does the study of neurodegenerative diseases help in the fight against Alzheimer’s?
The study of neurodegenerative diseases, including Alzheimer’s, is crucial as it enables researchers to identify common mechanisms that drive disease progression. Insights gained from diseases such as Huntington’s help inform our understanding of Alzheimer’s, allowing scientists to implement preventative strategies and therapeutic interventions targeted at shared pathologies.
| Key Point | Details |
|---|---|
| Microglial cells | Act as the brain’s immune system, clearing dead cells and pruning synapses. |
| Aberrant pruning | Can contribute to Alzheimer’s, Huntington’s, and other neurodegenerative disorders. |
| Impact of research | Stevens’ findings set a foundation for new treatments and early detection biomarkers for Alzheimer’s. |
| Funding sources | Research primarily supported by the National Institutes of Health (NIH). |
| Future implications | Research may significantly affect treatment for the nearly 7 million Americans with Alzheimer’s by 2050. |
Summary
Alzheimer’s research continues to evolve, particularly through the groundbreaking work by scientists like Beth Stevens. Her investigations into microglial cells have revealed critical insights into how the immune system functions in the brain and its relation to neurodegenerative diseases. By understanding the mechanisms behind aberrant pruning, new pathways have opened for the development of treatments and early diagnostics, ultimately aiming to improve the lives of millions affected by Alzheimer’s.