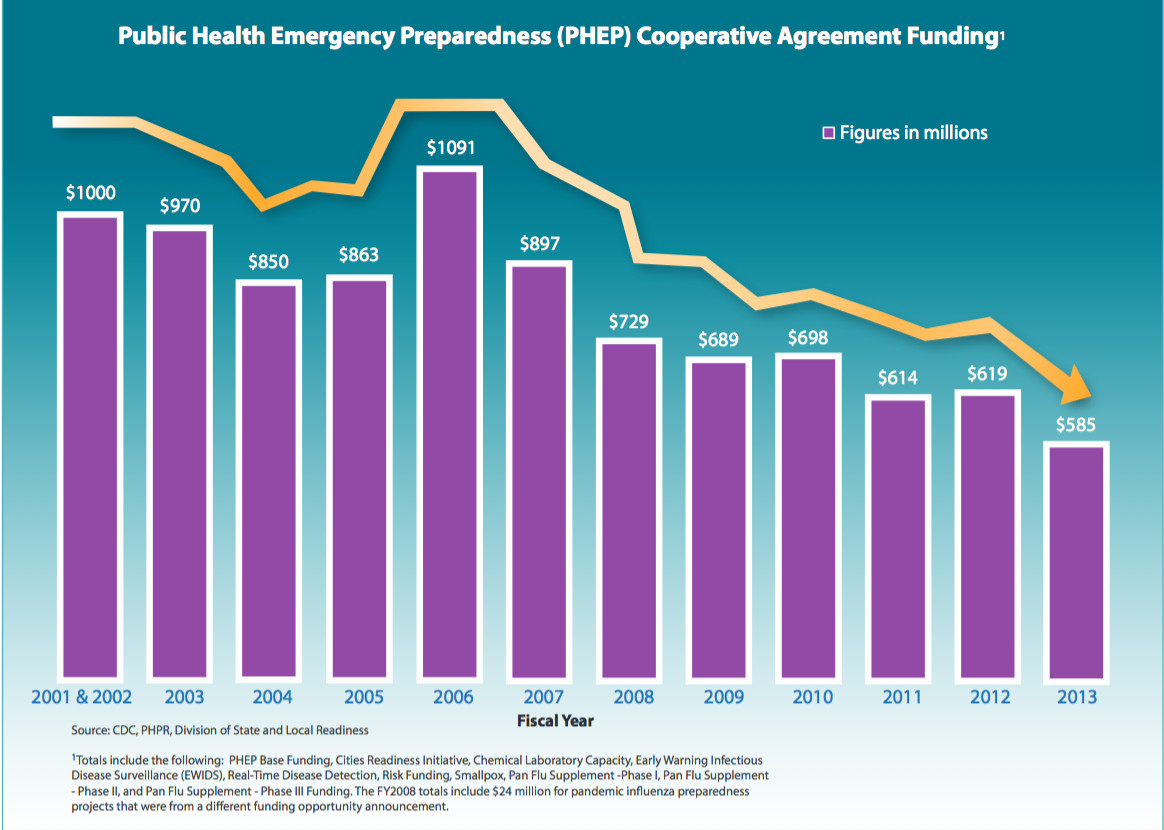
The impact of funding cuts on medical research is profound and far-reaching, fundamentally altering the landscape of how research is conducted. With significant reductions in medical research funding, like the recent NIH funding cuts, universities and research institutions face barriers that threaten the safety and rights of patients participating in clinical trials. An interruption in funding not only stalls ongoing research efforts but also jeopardizes the essential oversight needed to safeguard participant welfare, which is typically facilitated by Institutional Review Board (IRB) processes crucial for protecting vulnerable populations. As seen in the disruption at Harvard, these financial constraints can lead to halted studies, the suspension of collaborative efforts, and a loss of public trust in research initiatives. It’s critical to understand that every dollar dedicated to these research endeavors directly correlates with the health outcomes and innovations that impact our lives and communities.
When examining the repercussions of decreased financial support for biomedical investigations, one begins to grasp the extensive implications on patient safety and ethical oversight in clinical trials. The adverse effects stemming from diminished funding create a ripple effect across institutions involved in research, including major players like Harvard Medical School. Inadequate resources not only hinder the ability to conduct thorough reviews but also stifle the collaborative spirit necessary for tackling complex medical questions that require input from multiple sites. The loss of funding can derail teams dedicated to research ethics oversight and compliance, leaving researchers grappling with the challenges of conducting high-quality studies without the assurance of adequate participant protection. Ultimately, these funding issues compromise the integrity of advancements in healthcare research and may deter future contributions to medical innovation.
The Consequences of Funding Cuts on Medical Research
The recent funding cuts have far-reaching implications for the medical research landscape. As federal grants, such as those from the NIH, are crucial for facilitating various studies, the abrupt freeze of over $2 billion in federal research funding to institutions like Harvard signifies more than just monetary setbacks. These cuts pose a direct threat to ongoing clinical trials, limiting hospitals and universities’ abilities to conduct research that is essential for advancements in medical practices. With reduced resources, researchers may struggle to recruit participants, explore innovative treatments, and ultimately, enhance patient care.
Moreover, funding cuts impact not only the forefront of experimental treatments but also the essential oversight mechanisms central to patient safety, such as Institutional Review Boards (IRBs). The halt in funding means that many IRBs could face staffing reductions and resource constraints, which might impede their ability to adequately monitor research protocols and ensure compliance with ethical standards. This creates a cascading effect where the integrity of research practices erodes, putting patient welfare at risk.
The Role of Institutional Review Boards in Research Ethics Oversight
Institutional Review Boards (IRBs) serve as the cornerstone of research ethics oversight. Their primary function is to protect the rights and welfare of research participants by closely scrutinizing research proposals before approval, ensuring comprehensive informed consent protocols, and assessing risks of harm. In the context of medical research funding, IRBs uphold the ethical standards that direct institutions towards safe and responsible practices. They make it possible for researchers to conduct studies that ultimately lead to breakthrough medical advancements while safeguarding participants’ interests.
However, the effectiveness of IRBs can be jeopardized by funding cuts that limit proper training and operational capacity. As oversight becomes less robust due to diminished resources, the potential for ethical oversights increases. This scenario could exacerbate public mistrust of clinical research, especially in historically marginalized communities. Therefore, maintaining adequate funding for these ethical review boards is paramount to reinforcing the public’s trust and ensuring that research not only meets regulatory standards but surpasses them to genuinely protect individuals involved in medical studies.
Impact of Funding Cuts on Medical Research and Patient Safety
The impact of funding cuts extends beyond immediate financial considerations; it deeply affects patient safety and the integrity of medical research. When studies are halted or delayed due to lack of funding, it threatens the availability of critical treatments and therapies needed by patients. For example, ongoing research that aims to find solutions for chronic conditions may come to a standstill, leaving patients without alternative options. As noted previously, the SMART IRB system, essential for coordinating multi-site studies, has faced significant disruptions due to funding limitations, creating barriers for collaborative research efforts that are crucial for patient innovation.
The loss of federal funding for institutions like Harvard not only halts current projects but also stifles the potential for future research initiatives that could improve patient outcomes. When essential studies are impeded or canceled, it hinders the advancement of medical science and erodes public trust. Patients may become disillusioned with the clinical research process, fearing that ethical oversight and safety measures are compromised, ultimately deterring their participation in studies altogether. This vicious cycle emphasizes the urgent need for adequate funding to ensure sustained patient safety and ethical integrity in medical research.
The Historical Context of Research Ethics and Oversight
Understanding the historical context of research ethics is vital in recognizing the importance of oversight mechanisms like IRBs. The dark legacies of unethical practices, such as the Tuskegee Syphilis Study and other exploitative research, have shaped the stringent ethical standards we see today. These events revealed the dire consequences of neglecting patient rights and underscored the necessity for robust oversight to prevent future abuses. Consequently, regulations and oversight were implemented to safeguard the welfare of participants in medical research, ensuring that all studies are conducted with the utmost care and respect for individuals.
The establishment of IRBs was borne from these historical injustices, reflecting society’s commitment to ethical research practices. Funds allocated to support these boards are not just monetary investments; they are fundamental to preserving public trust in medical research. As we contemplate the implications of current funding cuts, we must remain mindful of this history and the critical lessons it imparts. Maintaining ethical oversight through adequate funding can help honor past victims and prevent a repeat of historical mistakes in contemporary research settings.
Navigating the Future of Medical Research Funding
The future landscape of medical research funding seems precarious, especially in light of recent government actions that have caused major disruptions. Researchers must navigate a complex web of financial constraints while striving to advance science and safeguard public health. Emphasizing alternative funding mechanisms may be necessary for institutions to mitigate the challenges posed by federal funding cuts. This could include fostering partnerships with private sector organizations, enhancing community engagement, and exploring philanthropic avenues to maintain research initiatives.
Additionally, public advocacy plays a crucial role in influencing policy decisions regarding research funding. Engaging the community and stakeholders to raise awareness about the importance of medical research can mobilize support for sustainable funding solutions. As health crises emerge and public health priorities shift, the demand for innovative research is ever-growing; hence, ensuring robust funding will be essential to meet these challenges head-on and preserve the integrity of medical advancements.
The Importance of Collaborative Research in Advancing Medicine
Collaboration among research institutions, hospitals, and universities significantly enhances the potential for groundbreaking medical advancements. By pooling resources and expertise, these entities can expedite the research cycle, share knowledge, and ultimately develop better healthcare solutions for patients. The SMART IRB system exemplifies the effectiveness of collaborative efforts in streamlining research oversight, allowing for more efficient management of multi-site studies and ensuring that patient safety and ethical standards are upheld.
However, the ongoing cuts to medical research funding threaten to undermine these collaborative efforts. When research agreements are stalled due to financial limitations, the momentum needed for significant medical breakthroughs suffers. Future treatments may take longer to develop, impacting patients’ access to effective therapies. Therefore, fostering an environment that encourages collaboration through reliable funding sources is vital to advance medical research and enhance patient care.
Maintaining Ethical Standards Amidst Financial Constraints
Amidst tightening budgets, the challenge of maintaining ethical standards in medical research becomes more pronounced. Funding cuts can lead to diminished oversight capabilities, which poses significant risks to patient safety and ethical compliance. In a climate where resources are scarce, researchers may inadvertently prioritize immediate financial needs over rigorous ethical oversight, jeopardizing the well-being of study participants. IRBs may also face challenges in providing sufficient training for members, exacerbating the difficulties in maintaining ethical integrity within research practices.
To counteract these effects, institutions must prioritize the allocation of available resources toward research ethics training and support for their IRB systems. This commitment is crucial in ensuring that individuals involved in medical research prioritize ethical practices even in the face of financial adversity. Upholding high ethical standards can be achieved through continued education, support, and public accountability, all fundamental for reinforcing trust between researchers and the communities they serve.
The Ripple Effects of Research Funding on Public Trust
The dynamics of research funding directly influence public trust in medical trials and clinical research. With the public increasingly aware of historical wrongdoings in research, transparency and ethical oversight are paramount in fostering a credible research environment. As funding cuts hinder the institutions’ abilities to carry out thorough oversight or maintain IRBs, public skepticism may arise, detaching communities from engagement in research initiatives. Patients may feel disillusioned if they perceive that the safeguards designed to protect them are weakened due to financial constraints.
Promoting an open dialogue about funding challenges and emphasizing continued commitments to research ethics can bridge the trust gap between researchers and the public. Encouraging community involvement and feedback as research initiatives unfold will foster a sense of partnership. By publicly demonstrating the importance of ethical standards and patient safety, researchers can help assure the public of their commitment to conducting responsible and transparent research, ultimately reinforcing trust.
Innovative Solutions for Sustaining Medical Research
To navigate the complex landscape of funding cuts in medical research, innovative solutions are essential. Embracing new funding models, such as public-private partnerships, could create opportunities for collaborations that benefit both sectors. Such partnerships can alleviate some of the financial burdens on academic institutions while allowing the private sector to contribute to advancements in medical science. Additionally, exploring crowdfunding platforms tailored for medical research could provide alternatives to traditional funding avenues, engaging the community in supporting vital studies.
Moreover, institutions can invest in capacity-building initiatives designed to enhance grant-seeking strategies among researchers. Providing training and resources can empower researchers to effectively pursue diverse funding sources, from non-profit organizations to international grants. Encouraging interdisciplinary collaborations can generate innovative ideas and attract funding from a broader array of stakeholders, ensuring that important medical research continues, regardless of fluctuations in federal funding.
Frequently Asked Questions
What is the impact of funding cuts on medical research funding and patient safety?
Funding cuts to medical research funding have dire consequences for patient safety and the integrity of clinical trials. Such cuts hinder the ability of Institutional Review Boards (IRBs) to effectively oversee research, leading to potential gaps in participant protection. The disruption in financial support can cause delays in ongoing studies and limit the capacity to initiate new research, ultimately compromising patient rights and safety.
How do NIH funding cuts affect research ethics oversight in medical studies?
NIH funding cuts severely undermine research ethics oversight by limiting resources available for institutional review boards (IRBs) that ensure compliance with ethical standards. These cuts can result in fewer personnel dedicated to monitoring studies, thus increasing the risk of ethical breaches and compromising the welfare of human participants involved in clinical research.
What are the consequences of NIH funding cuts on IRB patient safety protocols?
NIH funding cuts directly impact IRB patient safety protocols by restricting the financial and staffing resources needed to monitor and review medical studies adequately. The halt in funding can prevent IRBs from conducting thorough assessments of research proposals, which could put participants at risk and erode public trust in clinical research.
In what ways do funding cuts to Harvard medical research affect multi-site studies?
Funding cuts to Harvard medical research impede multi-site studies by disrupting the SMART IRB system, which streamlines research oversight across various institutions. Without necessary funding, new clinical sites cannot be added, which delays research progress and may negatively affect patient recruitment and overall study outcomes.
What impact do funding reductions have on the relationship between researchers and communities involved in medical research?
Funding reductions can exacerbate the mistrust between researchers and the communities they serve. When crucial funding for oversight and ethical compliance is cut, it can lead to perceptions of negligence in participant safety, discouraging community engagement and participation in future studies, ultimately affecting the advancement of medical research.
How do funding cuts threaten the advancements in medical research?
Funding cuts threaten advancements in medical research by postponing groundbreaking studies and clinical trials. Such disruptions can hinder collaboration between institutions and delay the development of innovative therapies, as researchers struggle with financial constraints, ultimately slowing the progress of potential treatments for diseases.
What role does research ethics oversight play in ensuring the safety of patients, especially under budget constraints?
Research ethics oversight is crucial for ensuring patient safety, particularly under budget constraints. Effective oversight by IRBs ensures compliance with ethical standards and safeguards participant welfare even amidst funding challenges. Ethical oversight helps maintain trust in medical research, fostering a commitment to prioritize patient rights despite financial limitations.
| Key Points | Details |
|---|---|
| Funding Cuts | The Trump administration froze over $2 billion in federal research grants to Harvard, affecting various research areas, including patient rights and safety. |
| Impact on IRBs | Institutional Review Boards (IRBs) are essential for overseeing medical research and ensuring participant safety, whose funding has been jeopardized. |
| Consequences of Halting Research | Funding cuts risk halting studies midstream, harming participants, entrenching public skepticism of research, and leading to delays in ongoing studies. |
| Historical Context | Failures in medical ethics and patient safety in the past emphasize the need for strict oversight by IRBs and continuous funding. |
| Ongoing Support | Despite funding cuts, Harvard Medical School continues to provide essential support to maintain collaborative research efforts nationwide. |
Summary
The impact of funding cuts on medical research is profound and multifaceted. The significant freeze in federal research funding has disrupted critical oversight mechanisms, specifically the essential role of Institutional Review Boards (IRBs), which are crucial in protecting the rights and safety of participants. As research studies face delays and halts, not only does this jeopardize the well-being of those involved, but it also risks eroding public trust in medical research initiatives. Moreover, historical examples highlight the dangers of inadequate oversight, reinforcing the need for reliable funding to ensure ethical standards and patient safety in clinical studies. Continued support and flexible funding solutions are imperative to uphold the integrity of medical research and reassure the community regarding their safety in participatory studies.